Oxygen Gas Production
Introduction
|
Oxygen gas makes up about 20% of the Earth's atmosphere. Higher concentrations of Oxygen gas increase the risk of fire and the combustion of flammable substances. One should keep this in mind and take appropriate safety precautions when working in an Oxygen-rich atmosphere.
This article will present two relatively simple methods of generating pure Oxygen gas, although other methods of production exist. The first method involves the decomposition of Hydrogen Peroxide using Manganese Dioxide as a catalyst. The second method employs a reaction between Hydrogen Peroxide and Sodium Hypochlorite ("bleach").
Method 1 - Hydrogen Peroxide Decomposition
Description
Hydrogen Peroxide (H2O2) will decompose rapidly in the presence of Manganese Dioxide, acting as the catalyst, to produce Oxygen gas and water.
![]()
When the Hydrogen Peroxide and Manganese Dioxide are mixed, Oxygen gas bubbles should be seen coming from the mixture. One can collect this Oxygen gas produced over water (through water-displacement) and store it inside a bottle for later use.
Materials
- Hydrogen Peroxide
- Manganese Dioxide / Manganese(IV) Oxide / MnO2
- Reaction Vessel (see below)
- Rubber Tubing
- Bucket or Large Bowl
- Collection Bottle (could be a simple plastic drinking bottle with lid)
- Water

Above: Manganese Dioxide - Catalyst for
the decomposition of Hydrogen Peroxide
Set-up
For the purposes of this experiment the reaction vessel shown in the pictures below was constructed using a flat-bottom flask, addition funnel, Claisen adapter, and a 90° hose adapter. A far less sophisticated reaction vessel will still work if the above pieces of glassware are not available.
In the above set up, the flow of Hydrogen Peroxide down from the addition funnel into the flat-bottom flask can be controlled via a stop-cock, so more H2O2 may be added as needed and some degree of control over the reaction can be achieved.
The gas flowing out of the reaction vessel through the attached rubber hose will be forced down beneath the water in the water-filled bowl. When the gas reaches the end of the tubing it will bubble out and float upward. An inverted, water-filled, bottle is positioned above the end of the tube will collects the gas bubbles. To aid in the collection of gas bubbles, one might consider using a small funnel placed in the mouth of the bottle. It is important to always keep the mouth of the inverted bottle below the water level in order to prevent the water from draining out and sucking air inside. As the gas bubbles accumulate at the top of the bottle they force the water out the bottom. If allowed to continue, the gas will eventually fill the entire bottle and all the water originally inside will be pushed out into the bowl. When the Oxygen generating reaction is complete, while keeping the mouth of the bottle submerged, reach under the water and screw the lid on.
As the Oxygen production reaction starts, the gas flowing out of the reaction vessel will be mostly air (presuming the flask was initially filled with air). As the reaction proceeds, the concentration of Oxygen gas inside the flask will increase and the bubbles flowing out of the tube will approach 100% Oxygen (but there may always be small traces of air remaining). Also, since the Oxygen is being generated using a “wet” production method and is being collected “over water”, the gas collected inside the bottle will be “wet”, meaning that it will contain a small amount of water vapor. The amount of water vapor contained in the collected gas depends on the temperature (which, in turn, determines the vapor pressure of water). To minimize water vapor in the collected gas, one should use a cool collection bath. Additionally, one may pass the gas through a drying tube (filled with a deliquescent material such as Calcium Chloride) before use.
Method 2 - Reaction between Hydrogen Peroxide and Bleach
Description
This method of production is very similar in many respects to the first method. The difference between the two is the chemicals involved in the process. The reaction may be performed in an identical apparatus to the first method and, again, the Oxygen gas produced may be collected over water for later use.
When Hydrogen Peroxide and Sodium Hypochlorite (NaOCl) are mixed, the two will react vigorously to produce Oxygen gas. The solutions need not be that concentrated to get a fast reaction, ordinary 3% H2O2 and ~6% household [Clorox] "beach" will be more than adequate to get a high relatively production rate. The only other products of the reaction are "salt" (Sodium Chloride) and water.
![]()
Materials
- Hydrogen Peroxide
- Bleach (must contain Sodium Hypochlorite; Clorox bleach or equivalent will work)
- Reaction Vessel
- Rubber Tubing
- Bucket or Large Bowl
- Collection Bottle
- Water
Set-up
The same set-up was used for the second method as was used for the first method of Oxygen production. For the second method, the addition funnel becomes much more important to dispense the liquids since the reaction proceeds very quickly once the H2O2 and bleach are mixed. A magnetic stirrer (and stir bar) was added to help mix the two liquids inside the flask, but the process will still work very well without one.
In the first method, one did not necessarily have a maximum amount of Oxygen which could be generated; one could always add more Hydrogen Peroxide to the reaction vessel (until the flask became full) where the H2O2 would be decomposed into water and Oxygen gas. However, in the second method, the amount of Oxygen gas which may be produced is constrained by the limiting reagent (which will be, in practice, the reactant initially placed in the flask). One will initially add some [fixed, finite] amount of either bleach of Hydrogen Peroxide (it does not matter which) into the flask; the additional funnel will then hold the second reagent. The additional funnel may be refilled mid-way through the reaction as many times as needed, so the reagent placed in the addition funnel can be added in excess while the reagent in the flask will limit the reaction and thus the amount of Oxygen gas which may be produced. One could, alternatively add a second addition funnel (which is easily done with a 3-necked Claisen adapter) to the apparatus, allowing one to add both H2O2 and bleach to the reaction vessel; in this case, one is then only limited by the volume of the flask in terms of how much Oxygen may be produced.
Oxygen gas Demonstrations
The following video demonstrates the vigorous decomposition of Hydrogen Peroxide using a Manganese Dioxide catalyst. In the video, a small amount of Manganese Dioxide is placed in a beaker. Approximately 35 mL of 30%, by weight, H2O2 solution is poured into the beaker. Immediately, the Hydrogen Peroxide begins to decompose, giving off Oxygen gas. The reaction is exothermic and a portion of the water is turned into steam, as seen in the video as a white mist coming off from the reaction (the Oxygen is invisible and is mixed in with the steam). The 30% Hydrogen Peroxide used for this demonstration is excessively concentrated for practical production needs. Using the ordinary, 3%, Hydrogen Peroxide sold in the pharmacy / grocery store will still work quite well for producing Oxygen gas.
|
Video
|
|---|
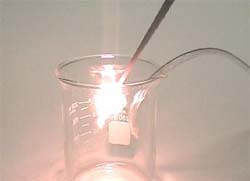
Increased concentrations of Oxygen gas in the atmosphere makes it easier for things to burn. In the following demonstration, a wood splint is lit on fire and then blown out so that the end is left smoldering (but not burning) in the ordinary air atmosphere. The smoldering splint is then inserted into a beaker filled with Oxygen gas (the small hose sticking out of the beaker is connected to the Oxygen generator and continually supplies a stream of O2). The increased concentration of Oxygen gas inside the beaker causes the smoldering splint to re-ignite and begin to burn brighter and faster than it did in the air atmosphere.
|
Video
|
|---|
Last updated:
03/12/2008