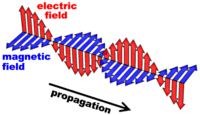
 Electromagnetic radiation is composed of a set of
orthogonally positioned electric and magnetic fields. Maxwell's
laws of electromagnetism state that a changing electric field will
generate a magnetic field, and, a changing magnetic field will induce
an electric field. These two oscillating fields form a self
propagating electromagnetic wave which, unlike ordinary waves requiring
a medium to propagate, is able to travel through a vacuum.
Electromagnetic radiation is composed of a set of
orthogonally positioned electric and magnetic fields. Maxwell's
laws of electromagnetism state that a changing electric field will
generate a magnetic field, and, a changing magnetic field will induce
an electric field. These two oscillating fields form a self
propagating electromagnetic wave which, unlike ordinary waves requiring
a medium to propagate, is able to travel through a vacuum.
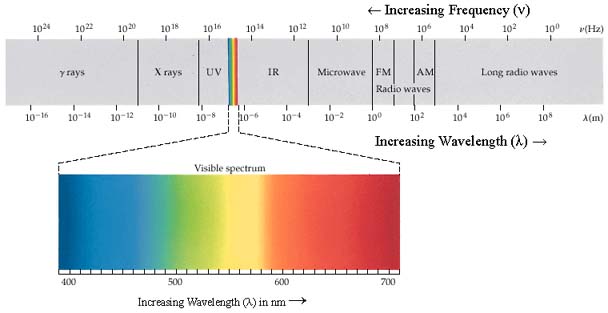
All visible light is a form of electromagnetic
radiation or energy carried in the form of electromagnetic waves. In
fact, visible light ![]() is just a very small part of the electromagnetic spectrum. Visible
light has very short wavelengths, between about 400 nanometers
and 700 nanometers. By comparison, Radio waves have much longer
wavelengths, on the order of several meters, whereas gamma rays can
have wavelengths smaller than 100 picometers.
is just a very small part of the electromagnetic spectrum. Visible
light has very short wavelengths, between about 400 nanometers
and 700 nanometers. By comparison, Radio waves have much longer
wavelengths, on the order of several meters, whereas gamma rays can
have wavelengths smaller than 100 picometers.
Every wave has an energy associated with it which is carried in discrete packets or "quanta" of energy called photons. The photon's energy (E) can be related in terms of the speed of light in a vacuum (c), the wavelength (λ), and Planks constant (h).
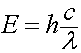
h = 6.63 x10-34 J s / photon
c = 299,792,458 m/s
Although a photon has no mass and zero electric charge, its energy can influence electrons surrounding an atom. When light is shown on an object, it is possible for the electrons to absorb energy from the light in the form of a photon, increasing the electron's energy. Electrons surrounding an atom can only exist within specific energy levels; therefore if the photon carries sufficient energy, the electron can be forced to jump into a higher energy level, or be ejected out altogether and become a free particle.
When an electron moves up to a higher energy level, the atom is said to be in the "excited" state. However, this state of excitement will not last long. Electrons have a natural tendency to try to find the lowest energy state possible, known as the "ground" state. At its first opportunity, the electron will drop down into a lower orbital position and with it, give up its extra energy in the form of a photon.
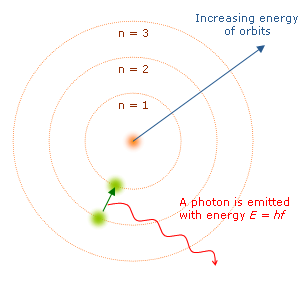 The emitted photon will carry away specific
amounts of energy from the atom depending on the number of energy
levels the excited electron drops. There are multiple paths for
the electron to take depending on the amount of energy being absorbed
or released. Take the simplest atom, Hydrogen, with only one
electron. Depending on the energy that Hydrogen's one electron
absorbs, the electron may jump one or more energy levels at a time,
afterward, the electron may return to the ground state by releasing a
single photon, or by releasing multiple photons and stopping
temporarily in other orbitals. The multiple paths Hydrogen's
electron may take each represent a photon with a different frequency
and carrying a different amount of energy.
The emitted photon will carry away specific
amounts of energy from the atom depending on the number of energy
levels the excited electron drops. There are multiple paths for
the electron to take depending on the amount of energy being absorbed
or released. Take the simplest atom, Hydrogen, with only one
electron. Depending on the energy that Hydrogen's one electron
absorbs, the electron may jump one or more energy levels at a time,
afterward, the electron may return to the ground state by releasing a
single photon, or by releasing multiple photons and stopping
temporarily in other orbitals. The multiple paths Hydrogen's
electron may take each represent a photon with a different frequency
and carrying a different amount of energy.
The energy of a photon released by an excited Hydrogen atom can be calculated by finding the difference in energy levels between the initial and final energy levels of the atom given by the following equation,
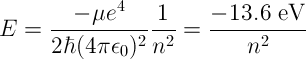
μ is the reduced mass of the electron (approximately equal to electron
rest mass),
ħ is the reduced Plank constant (ħ = h / 2π),
ϵ0 is the electric permittivity of free space,
n is the principle quantum number.
For n = 1, one obtains the ground state energy of the hydrogen atom, -13.6 eV.
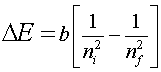
This calculation, however, only works due to the
simplicity of the one electron system; it fails for all other atoms
with multiple electrons.
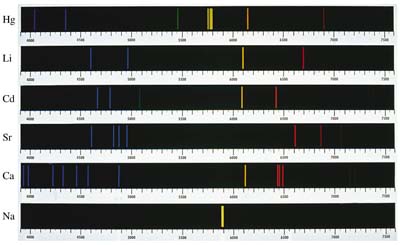
Light in all the various wavelengths emitted by an atom make up the
atomic emission spectrum. The emission spectrum is made up of
bands of light corresponding to the varying wavelengths of photons
emitted by the atom. By contrast, the atomic absorption spectrum
shows at which wavelengths of light that atom will absorb
photons.
Each element has a unique line spectrum at which it absorbs or emits photons. Atoms do not necessarily only absorb or emit photons in the visible range, many elements have spectral lines in the infrared or ultraviolet wavelengths.
By studying these spectral lines, it is possible to identify certain elements in an object due to its characteristic spectral patterns.
Electrons can also be excited in other ways, for instance by heating the object up in a flame or by an electric current. Neon lights work on the principle of an electric current exciting the electrons in a gas. When the electrons return to the ground state, they emit a photon. To create the desired color of light, a variety of gasses are used. To create yellow light, Sodium vapor is used whereas the classic red light is produced with Neon gas.
Last updated: 07/11/2011