Sulfuric Acid
Sulfuric Acid is a clear, polar, diprotic acid which has widespread use in industry as well as in chemical synthesis and analysis.
Sulfuric Acid undergoes two disassociation reactions, producing H+ ions in solution.
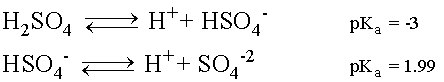
|
Sulfuric Acid is a strong acid and the first disassociation
will readily occur so much so that practically all of the H2SO4
will break up into H+ and Bisulfate (HSO4-1)
ions in solution.
The Bisulfate ion can then disassociate further giving off
another H+ ion and leaving a Sulfate ion (SO4-2)
in solution. However, being a weak acid, the equilibrium for
the second disassociation favors Bisulfate formation over H+
and SO4-2.
Sulfuric
Acid is special in that it can act both as an ordinary acid or
an oxidizing acid depending on its temperature and
concentration.
Cool, dilute solutions of Sulfuric acid will react with metals
above Hydrogen on the activity series to produce a Sulfate
salt and release Hydrogen gas.
For example, the reaction between cool, dilute Sulfuric Acid and Zinc metal (assuming complete double- disassociation of the H2SO4) is:
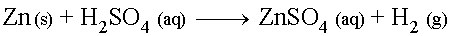
A hot, concentrated solution of Sulfuric acid can act as an oxidizing acid, allowing the acid to react with materials which would ordinarily be unreactive to acids, such as Copper metal. In the process, the Copper metal is oxidized and the acid is reduced, forming a solution of Copper(II) Sulfate salt, water, and releasing Sulfur Dioxide (SO2) fumes, instead of the Hydrogen gas one might expect when reacting acids with metals.
Sulfuric Acid is a power desiccant and the absorption of water is highly thermodynamically favorable, releasing a considerable amount of energy in the form of heat. A Sulfuric Acid solution’s temperature will rise when diluted so one must remember to “Always Add Acid” (AAA) to help avoid the hazard of the Sulfuric Acid’s temperature rising too rapidly, causing the solution to boil and potentially spattering acid out of the container. Always, slowly, pour acid into cold water when diluting. Sulfuric Acid is such a power desiccant that it will absorb moisture from its surroundings and the air to dilute itself and will even dehydrate other molecules in order to absorb the water.

Due to its highly corrosive nature and potential oxidizing properties, Sulfuric acid can be extremely dangerous when not handled responsibly. Always wear proper protective clothing when working around Sulfuric Acid (including, but not limited to, gloves and eye protection), and practice appropriate safety precautions.
The video below depicts the necessity of wearing appropriate protective clothing when working with Sulfuric Acid. A, black colored, concentrated Sulfuric Acid solution is poured onto a white T-Shirt. In just moments, the T-Shirt is eaten away by the acid and would have provided no real protection to the wearer. Video is played at 2X speed.
|
Video
|
|---|
Sulfuric Acid’s dehydrating property is illustrated below as the acid rips out Hydrogen and Oxygen atoms from a sugar molecule to form water which it then absorbs. The Sucrose, sugar, molecule is converted into a Carbon sludge as it is dehydrated by the acid. The top video is played at 20X speed. The middle and bottom videos are played at 2X speed. The bottom video employs a higher concentration of Sulfuric acid (>95% H2SO4 by weight) than that used in the previous two videos. The sugar is converted into a black Carbon goo which expands to fill (and overflow from) the container due to the pockets of steam trapped inside.
|
Video
|
|---|
|
Last updated:
06/01/2008








